Objectives of the Study
In 2017, Rare Patient Voice funded and conducted a market research survey on the perceptions of advocacy groups for patients with rare diseases and their roles in the recovery and treatment process. The researchers also wanted to learn the opinions of caregivers and patients regarding the partnership of advocacy groups in pharmaceutical company activities.
The questions covered three main areas; namely, the role of advocacy groups in the diagnosis and treatment of patients, the performance of advocacy groups in realizing their respective goals, and the advantages and disadvantages of partnerships between advocacy groups and pharmaceutical companies.
The goal of the study was to shed light on the level of involvement and support that advocacy groups offer to patients and caregivers. It also aimed to identify issues that prevent these groups from fulfilling their advocacies and the solutions that can help them get past those challenges.
Summary of the Study
The survey had 3,455 respondents (2,344 patients; 757 caregivers; 326 patients and caregivers, and 28 who were neither), and was carried out from March 30 to April 7, 2017. All of the researchers and respondents were from the United States.
From this pool of respondents, the researchers were able to get feedback from individuals representing over 150 diseases (patients or caregivers of patients diagnosed with over 150 rare diseases) and over 800 advocacy groups.
The survey was conducted in three parts:
On Involvement with Advocacy Groups
Of the 3,341 respondents who answered the question on whether they were involved with an advocacy group that supports patients with rare diseases, 46% said yes and 54% said no.
For the follow-up question on why they did not work with an advocacy group, only 1,825 respondents answered. Among them, 48.5% admitted that they did not know of any relevant group (relating to their or a loved one's illness). Twenty-six percent said they didn't have time to look for one, while 19.5% had other existing support options and did not find the need to look for advocacy groups.
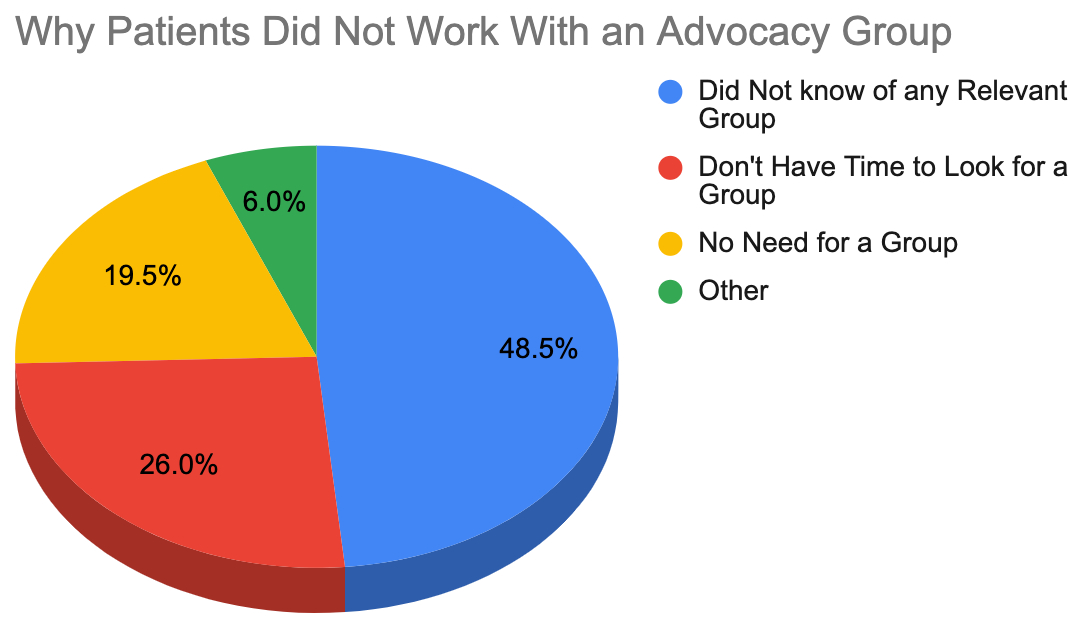
Curiously, 17.2% of the respondents for this question felt that working with advocacy groups was inconvenient, and 12.1% said they would rather face the challenges of treatment and providing care for patients with rare diseases on their own.
The same question also revealed some issues that a few people had with advocacy groups. Over five percent believed they do not give the support that patients and caregivers need; 1.2% did not agree with the goals of the advocacy group they know of, and 0.9% had personality clashes with an advocacy group's staff.
On Advocacy Group Performance
Most people acknowledged the importance of advocacy groups in spreading awareness and information about rare diseases. Out of the 3,016 who answered this question, 94% believed they were helpful to the patients, and 93% said that they were reliable sources of information for the patients and their families.
The majority of the survey's respondents also acknowledged that advocacy groups could help educate the public about specific illnesses; help patients find specialists and doctors; provide information about ongoing clinical trials, and advocate with the government regarding the patients' welfare.
On Partnerships with Biopharmaceuticals
When asked if they were in favor of advocacy groups working with biotech companies in developing drugs for rare diseases, 55.1% of the respondents said they were in favor. Only 9.7% opposed, while 35.2% were unsure about the matter.
Those who were in favor were driven by their need to find an effective treatment for their own or their loved one's illness. They also believed that advocacy groups were in the best position to help biotech companies because they knew what patients needed in the course of their treatment. The knowledge that these partnerships could also drive down the cost of treatment for patients was another factor that made these partnerships favorable.
On the other hand, the reasons behind the opposition were varied. Some insisted that advocacy groups should have no biases about treatment options. Others were worried that corporate greed overpowered all efforts of drug development, turning scientific endeavors into profit-generating schemes. Nevertheless, only 12% of 2,867 respondents said that partnerships between advocacy groups and pharmaceutical companies had not been successful at all. Thirty-eight percent believed they were "somewhat successful," while 50% were undecided.
Findings and Recommendations
The findings of the study revealed that even though advocacy groups for patients with rare diseases were being credited for their efforts in raising awareness about rare diseases in the community, many were still unaware of them and the aid they could give to patients and caregivers.
Besides raising awareness about their goals, support organizations have to exert more effort in informing the community about their role in medical drug development. They also need to reassure patients and caregivers that their motivations for partnering with biotech companies are not profit-driven; that they will remain impartial and independent, and that the patients' well-being will be their priority.






